Seznamy 158 Structure Of Atom Of Nitrogen
Seznamy 158 Structure Of Atom Of Nitrogen. There are many things to learn when we draw n 2 lewis structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Seven electrons (white) occupy available electron shells (rings).
Prezentováno Atom Wikipedia
Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 7), the most common isotope of the element nitrogen.0.75å · cross section (thermal neutron capture) a /barns:
0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns:

17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings).

Seven electrons (white) occupy available electron shells (rings). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings).

17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

There are many things to learn when we draw n 2 lewis structure... 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Seven electrons (white) occupy available electron shells (rings). 0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen.

Structure of nitrogen · atomic radius: There are many things to learn when we draw n 2 lewis structure. Seven electrons (white) occupy available electron shells (rings). 0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. 7), the most common isotope of the element nitrogen.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 7), the most common isotope of the element nitrogen.. 0.75å · cross section (thermal neutron capture) a /barns:

7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 0.75å · cross section (thermal neutron capture) a /barns:

17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

There are many things to learn when we draw n 2 lewis structure... . 7), the most common isotope of the element nitrogen.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. Seven electrons (white) occupy available electron shells (rings).

0.75å · cross section (thermal neutron capture) a /barns: Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Structure of nitrogen · atomic radius: Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius:

Structure of nitrogen · atomic radius:. Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen.

7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure. Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 7), the most common isotope of the element nitrogen. Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns:

17.3cm 3 /mol · covalent radius:. 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure.

The nucleus consists of 7 protons (red) and 7 neutrons (orange).. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: Structure of nitrogen · atomic radius: There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

17.3cm 3 /mol · covalent radius:.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

There are many things to learn when we draw n 2 lewis structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Structure of nitrogen · atomic radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange).

7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The nucleus consists of 7 protons (red) and 7 neutrons (orange).. The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius:. 7), the most common isotope of the element nitrogen.

0.75å · cross section (thermal neutron capture) a /barns:. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 7), the most common isotope of the element nitrogen. Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure. Seven electrons (white) occupy available electron shells (rings). Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. There are many things to learn when we draw n 2 lewis structure.

17.3cm 3 /mol · covalent radius:. 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure... Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms... Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure.

17.3cm 3 /mol · covalent radius:. . There are many things to learn when we draw n 2 lewis structure.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

7), the most common isotope of the element nitrogen.. 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.
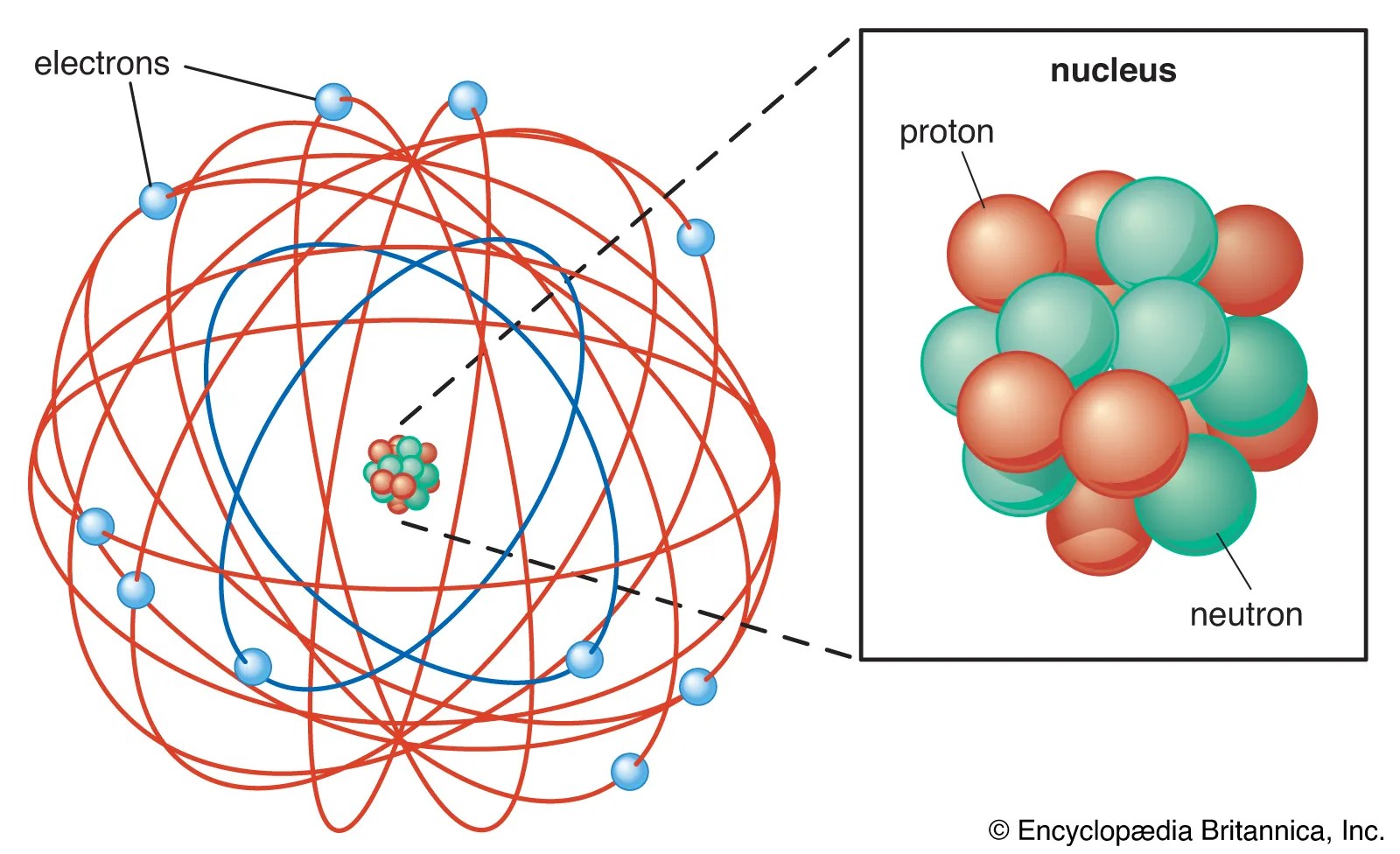
Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure.. 17.3cm 3 /mol · covalent radius:

Structure of nitrogen · atomic radius:.. 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen.. 17.3cm 3 /mol · covalent radius:

Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Structure of nitrogen · atomic radius:.. 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Structure of nitrogen · atomic radius: Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. 0.75å · cross section (thermal neutron capture) a /barns:

The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. Seven electrons (white) occupy available electron shells (rings).

17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Structure of nitrogen · atomic radius:

Structure of nitrogen · atomic radius:. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. 7), the most common isotope of the element nitrogen.

0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Seven electrons (white) occupy available electron shells (rings).
Nitrogen is a diatomic molecule and contains only two nitrogen atoms... 0.75å · cross section (thermal neutron capture) a /barns: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 7), the most common isotope of the element nitrogen... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure. Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Seven electrons (white) occupy available electron shells (rings). Seven electrons (white) occupy available electron shells (rings).

There are many things to learn when we draw n 2 lewis structure.. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings)... Structure of nitrogen · atomic radius:

Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: Structure of nitrogen · atomic radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Seven electrons (white) occupy available electron shells (rings).. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.
0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Seven electrons (white) occupy available electron shells (rings).
The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange)... 0.75å · cross section (thermal neutron capture) a /barns:
Seven electrons (white) occupy available electron shells (rings). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. Structure of nitrogen · atomic radius:. 0.75å · cross section (thermal neutron capture) a /barns:
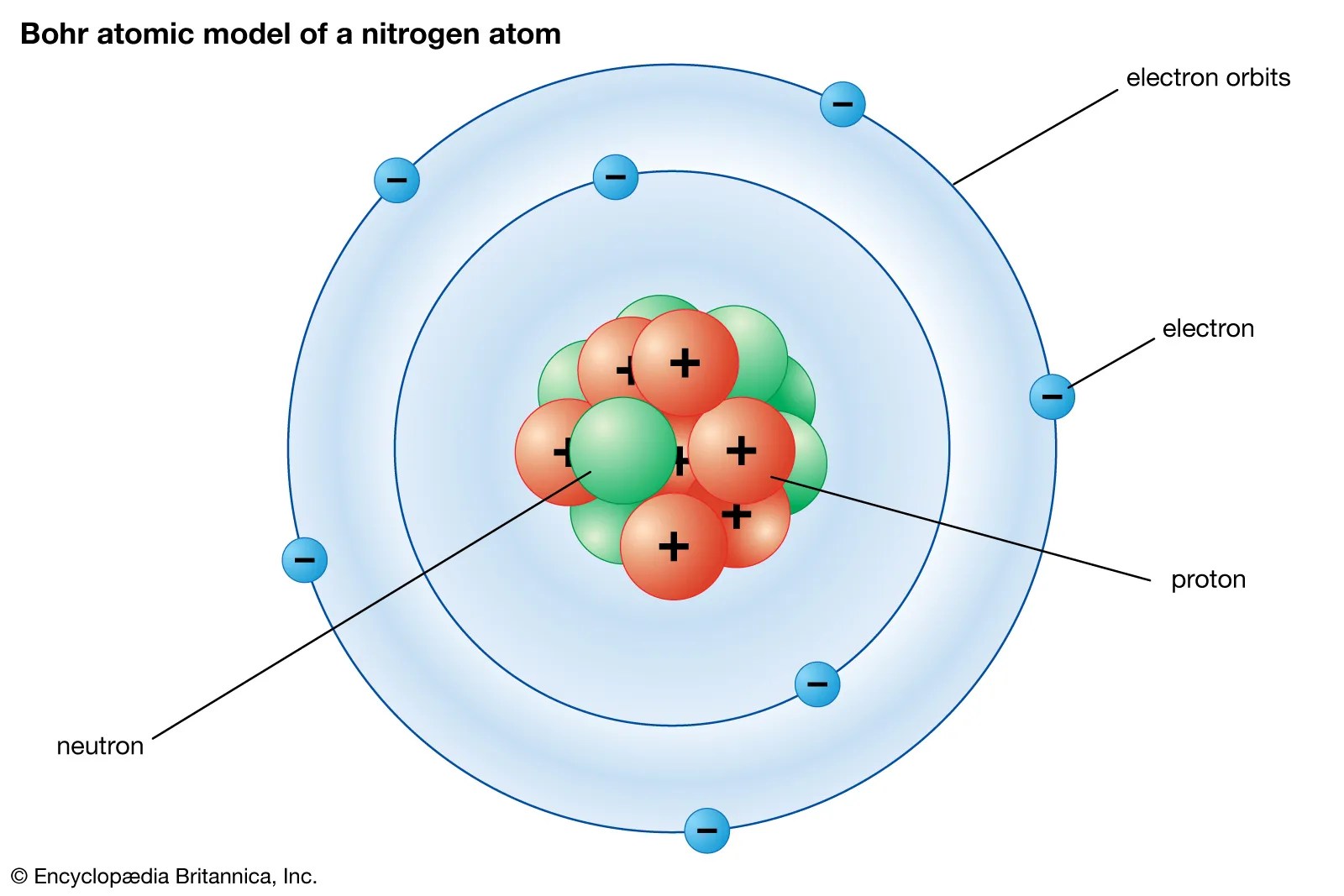
Seven electrons (white) occupy available electron shells (rings)... Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.
7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure. Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

17.3cm 3 /mol · covalent radius:. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Nitrogen is a diatomic molecule and contains only two nitrogen atoms.
7), the most common isotope of the element nitrogen. 7), the most common isotope of the element nitrogen. Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings).. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Structure of nitrogen · atomic radius:.. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius:.. 7), the most common isotope of the element nitrogen.

There are many things to learn when we draw n 2 lewis structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). 0.75å · cross section (thermal neutron capture) a /barns: Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius:. 7), the most common isotope of the element nitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (orange)... The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. Structure of nitrogen · atomic radius: Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 17.3cm 3 /mol · covalent radius:. 7), the most common isotope of the element nitrogen.
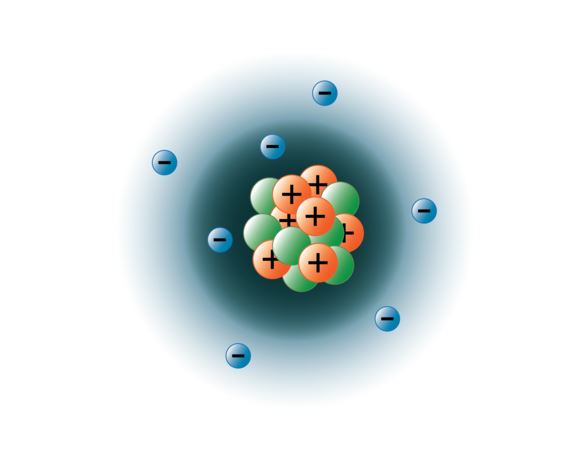
Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen.. Structure of nitrogen · atomic radius:

The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

17.3cm 3 /mol · covalent radius:. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings). 0.75å · cross section (thermal neutron capture) a /barns: 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 17.3cm 3 /mol · covalent radius:

Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: There are many things to learn when we draw n 2 lewis structure.. 0.75å · cross section (thermal neutron capture) a /barns:

Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Structure of nitrogen · atomic radius:

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure.. 7), the most common isotope of the element nitrogen.

7), the most common isotope of the element nitrogen... Seven electrons (white) occupy available electron shells (rings). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Structure of nitrogen · atomic radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns:. There are many things to learn when we draw n 2 lewis structure.

There are many things to learn when we draw n 2 lewis structure... The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings)... 17.3cm 3 /mol · covalent radius:

The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: 7), the most common isotope of the element nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Nitrogen is a diatomic molecule and contains only two nitrogen atoms... 17.3cm 3 /mol · covalent radius:

17.3cm 3 /mol · covalent radius: 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns:. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

7), the most common isotope of the element nitrogen. 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms... 7), the most common isotope of the element nitrogen.

Structure of nitrogen · atomic radius: There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms... Structure of nitrogen · atomic radius:

There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure.. Seven electrons (white) occupy available electron shells (rings).

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: Structure of nitrogen · atomic radius:

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Structure of nitrogen · atomic radius:. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

There are many things to learn when we draw n 2 lewis structure. There are many things to learn when we draw n 2 lewis structure. Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 0.75å · cross section (thermal neutron capture) a /barns:

17.3cm 3 /mol · covalent radius:. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius:
The nucleus consists of 7 protons (red) and 7 neutrons (orange). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). Nitrogen is a diatomic molecule and contains only two nitrogen atoms... There are many things to learn when we draw n 2 lewis structure.

There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. There are many things to learn when we draw n 2 lewis structure.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Seven electrons (white) occupy available electron shells (rings). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Structure of nitrogen · atomic radius:

17.3cm 3 /mol · covalent radius:. Structure of nitrogen · atomic radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

Structure of nitrogen · atomic radius: Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings).

17.3cm 3 /mol · covalent radius:. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Structure of nitrogen · atomic radius: Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange)... 0.75å · cross section (thermal neutron capture) a /barns:

There are many things to learn when we draw n 2 lewis structure.. Structure of nitrogen · atomic radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings)... Nitrogen is a diatomic molecule and contains only two nitrogen atoms.
