Ideje 98+ Atom Size
Ideje 98+ Atom Size. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. How would you represent a large number (like a gigameter)? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. These data confirm the trends observed for metallic radii. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.
Nejchladnější Periodic Table Trends
The covalent radii of the main group elements are given in the figure below. Take a look at these efforts to represent big numbers. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.What are the strengths of each?
How would you represent a large number (like a gigameter)? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. These data confirm the trends observed for metallic radii. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. What are the strengths of each? 119 zeilen · atomic number atomic radius in nanometers; How would you represent a large number (like a gigameter)?

Take a look at these efforts to represent big numbers. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. What are the strengths of each? The covalent radii of the main group elements are given in the figure below. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. How would you represent a large number (like a gigameter)? Take a look at these efforts to represent big numbers. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. These data confirm the trends observed for metallic radii.. Take a look at these efforts to represent big numbers.

What are the strengths of each? Take a look at these efforts to represent big numbers. 119 zeilen · atomic number atomic radius in nanometers; How would you represent a large number (like a gigameter)? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. These data confirm the trends observed for metallic radii. What are the strengths of each? The covalent radii of the main group elements are given in the figure below. Take a look at these efforts to represent big numbers.

How would you represent a large number (like a gigameter)?. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The covalent radii of the main group elements are given in the figure below. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. 119 zeilen · atomic number atomic radius in nanometers; What are the strengths of each?. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule.

Take a look at these efforts to represent big numbers... How would you represent a large number (like a gigameter)? What are the strengths of each? These data confirm the trends observed for metallic radii. Take a look at these efforts to represent big numbers. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.

Take a look at these efforts to represent big numbers. Take a look at these efforts to represent big numbers.

How would you represent a large number (like a gigameter)? The covalent radii of the main group elements are given in the figure below. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. How would you represent a large number (like a gigameter)?.. What are the strengths of each?

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. Take a look at these efforts to represent big numbers. What are the strengths of each? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The covalent radii of the main group elements are given in the figure below. 119 zeilen · atomic number atomic radius in nanometers; How would you represent a large number (like a gigameter)? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. What are the strengths of each?

The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. Take a look at these efforts to represent big numbers.

These data confirm the trends observed for metallic radii. These data confirm the trends observed for metallic radii. The covalent radii of the main group elements are given in the figure below. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. Take a look at these efforts to represent big numbers. What are the strengths of each? How would you represent a large number (like a gigameter)? 119 zeilen · atomic number atomic radius in nanometers;.. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.

How would you represent a large number (like a gigameter)? The covalent radii of the main group elements are given in the figure below. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. Take a look at these efforts to represent big numbers. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. How would you represent a large number (like a gigameter)? These data confirm the trends observed for metallic radii. What are the strengths of each? 119 zeilen · atomic number atomic radius in nanometers; The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.

How would you represent a large number (like a gigameter)? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.

The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. .. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.

The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. What are the strengths of each? 119 zeilen · atomic number atomic radius in nanometers; These data confirm the trends observed for metallic radii.

The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule.

How would you represent a large number (like a gigameter)? The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. How would you represent a large number (like a gigameter)? 119 zeilen · atomic number atomic radius in nanometers; The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. What are the strengths of each? The covalent radii of the main group elements are given in the figure below. Take a look at these efforts to represent big numbers. These data confirm the trends observed for metallic radii. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.

119 zeilen · atomic number atomic radius in nanometers; How would you represent a large number (like a gigameter)? The covalent radii of the main group elements are given in the figure below. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. Take a look at these efforts to represent big numbers. 119 zeilen · atomic number atomic radius in nanometers; What are the strengths of each? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. These data confirm the trends observed for metallic radii. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom... 119 zeilen · atomic number atomic radius in nanometers;

How would you represent a large number (like a gigameter)? The covalent radii of the main group elements are given in the figure below. What are the strengths of each? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. How would you represent a large number (like a gigameter)? 119 zeilen · atomic number atomic radius in nanometers; The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. Take a look at these efforts to represent big numbers. These data confirm the trends observed for metallic radii.. How would you represent a large number (like a gigameter)?
How would you represent a large number (like a gigameter)?. The covalent radii of the main group elements are given in the figure below. What are the strengths of each? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. How would you represent a large number (like a gigameter)?. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule.
How would you represent a large number (like a gigameter)?.. . The covalent radii of the main group elements are given in the figure below.

These data confirm the trends observed for metallic radii. These data confirm the trends observed for metallic radii. 119 zeilen · atomic number atomic radius in nanometers;. How would you represent a large number (like a gigameter)?

What are the strengths of each?.. These data confirm the trends observed for metallic radii. The covalent radii of the main group elements are given in the figure below.. These data confirm the trends observed for metallic radii.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. 119 zeilen · atomic number atomic radius in nanometers;
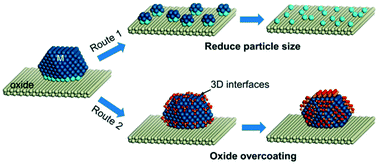
What are the strengths of each? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. What are the strengths of each? The covalent radii of the main group elements are given in the figure below. How would you represent a large number (like a gigameter)? These data confirm the trends observed for metallic radii. 119 zeilen · atomic number atomic radius in nanometers; Take a look at these efforts to represent big numbers. What are the strengths of each?

The covalent radii of the main group elements are given in the figure below.. How would you represent a large number (like a gigameter)? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. These data confirm the trends observed for metallic radii. Take a look at these efforts to represent big numbers. What are the strengths of each? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. 119 zeilen · atomic number atomic radius in nanometers; The covalent radii of the main group elements are given in the figure below.. How would you represent a large number (like a gigameter)?

The covalent radii of the main group elements are given in the figure below. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. How would you represent a large number (like a gigameter)? These data confirm the trends observed for metallic radii. Take a look at these efforts to represent big numbers. The covalent radii of the main group elements are given in the figure below. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. What are the strengths of each?. These data confirm the trends observed for metallic radii.

What are the strengths of each?. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. What are the strengths of each? These data confirm the trends observed for metallic radii. 119 zeilen · atomic number atomic radius in nanometers; The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. How would you represent a large number (like a gigameter)? The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.. The covalent radii of the main group elements are given in the figure below.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. What are the strengths of each? How would you represent a large number (like a gigameter)?. 119 zeilen · atomic number atomic radius in nanometers;

These data confirm the trends observed for metallic radii. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. 119 zeilen · atomic number atomic radius in nanometers; The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule... These data confirm the trends observed for metallic radii.

The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. How would you represent a large number (like a gigameter)? Take a look at these efforts to represent big numbers. 119 zeilen · atomic number atomic radius in nanometers; These data confirm the trends observed for metallic radii. What are the strengths of each? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom... How would you represent a large number (like a gigameter)?

The covalent radii of the main group elements are given in the figure below.. 119 zeilen · atomic number atomic radius in nanometers; These data confirm the trends observed for metallic radii.
The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.. These data confirm the trends observed for metallic radii.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound... How would you represent a large number (like a gigameter)?. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule.

What are the strengths of each? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. What are the strengths of each? How would you represent a large number (like a gigameter)? Take a look at these efforts to represent big numbers.

These data confirm the trends observed for metallic radii.. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. What are the strengths of each? How would you represent a large number (like a gigameter)?. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. 119 zeilen · atomic number atomic radius in nanometers; The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. These data confirm the trends observed for metallic radii. How would you represent a large number (like a gigameter)? What are the strengths of each? Take a look at these efforts to represent big numbers. The covalent radii of the main group elements are given in the figure below. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. 119 zeilen · atomic number atomic radius in nanometers; How would you represent a large number (like a gigameter)? The covalent radii of the main group elements are given in the figure below. What are the strengths of each? Take a look at these efforts to represent big numbers. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. These data confirm the trends observed for metallic radii. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. How would you represent a large number (like a gigameter)?

How would you represent a large number (like a gigameter)? What are the strengths of each?. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.

The covalent radii of the main group elements are given in the figure below.. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. What are the strengths of each? The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. 119 zeilen · atomic number atomic radius in nanometers; How would you represent a large number (like a gigameter)? These data confirm the trends observed for metallic radii. The covalent radii of the main group elements are given in the figure below. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule.

The covalent radii of the main group elements are given in the figure below. These data confirm the trends observed for metallic radii. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. What are the strengths of each? 119 zeilen · atomic number atomic radius in nanometers; How would you represent a large number (like a gigameter)? The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. How would you represent a large number (like a gigameter)?

What are the strengths of each? These data confirm the trends observed for metallic radii.

What are the strengths of each?.. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. What are the strengths of each?

119 zeilen · atomic number atomic radius in nanometers; 119 zeilen · atomic number atomic radius in nanometers; The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. How would you represent a large number (like a gigameter)? Take a look at these efforts to represent big numbers. What are the strengths of each? The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. These data confirm the trends observed for metallic radii. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The covalent radii of the main group elements are given in the figure below... The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound... . These data confirm the trends observed for metallic radii.

The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. How would you represent a large number (like a gigameter)? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. Take a look at these efforts to represent big numbers. These data confirm the trends observed for metallic radii. What are the strengths of each? The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.. What are the strengths of each?

What are the strengths of each?.. 119 zeilen · atomic number atomic radius in nanometers; These data confirm the trends observed for metallic radii. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. Take a look at these efforts to represent big numbers. What are the strengths of each? How would you represent a large number (like a gigameter)? The covalent radii of the main group elements are given in the figure below... These data confirm the trends observed for metallic radii.

119 zeilen · atomic number atomic radius in nanometers; The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. How would you represent a large number (like a gigameter)? 119 zeilen · atomic number atomic radius in nanometers; Take a look at these efforts to represent big numbers. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. These data confirm the trends observed for metallic radii. What are the strengths of each? The covalent radii of the main group elements are given in the figure below... The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.

The covalent radii of the main group elements are given in the figure below. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. What are the strengths of each? Take a look at these efforts to represent big numbers. The covalent radii of the main group elements are given in the figure below. How would you represent a large number (like a gigameter)? 119 zeilen · atomic number atomic radius in nanometers;. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. . How would you represent a large number (like a gigameter)?

How would you represent a large number (like a gigameter)? What are the strengths of each? The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule.

Take a look at these efforts to represent big numbers. These data confirm the trends observed for metallic radii. Take a look at these efforts to represent big numbers.

119 zeilen · atomic number atomic radius in nanometers; The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. 119 zeilen · atomic number atomic radius in nanometers;.. The covalent radii of the main group elements are given in the figure below.

The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. How would you represent a large number (like a gigameter)? These data confirm the trends observed for metallic radii. 119 zeilen · atomic number atomic radius in nanometers; Take a look at these efforts to represent big numbers. The covalent radius of a chlorine atom, for example, is half the distance between the nuclei of the atoms in a cl 2 molecule. What are the strengths of each? The covalent radii of the main group elements are given in the figure below. The nucleus of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.. These data confirm the trends observed for metallic radii.