Chiral Atom Examples Vynikající
Chiral Atom Examples Vynikající. The most common type of stereogenic element is a stereogenic center, or stereocenter. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.
Nejchladnější Examples Of The Types Of Chirality A D And Special Cases E F Download Scientific Diagram
A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. The lack of a plane of symmetry makes the carbon chiral. Chiral molecules will usually have a stereogenic element from which chirality arises. However, the use of chiral chemical catalysts is usually costly. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.And then we'll see examples that one or both of these are true.
Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. The higher the atomic number, the higher the priority.

The lack of a plane of symmetry makes the carbon chiral. However, the use of chiral chemical catalysts is usually costly. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center... In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.

What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. And then we'll see examples that one or both of these are true. The lack of a plane of symmetry makes the carbon chiral. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. Let's say it's bonded to a …. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below.

The lack of a plane of symmetry makes the carbon chiral. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number.
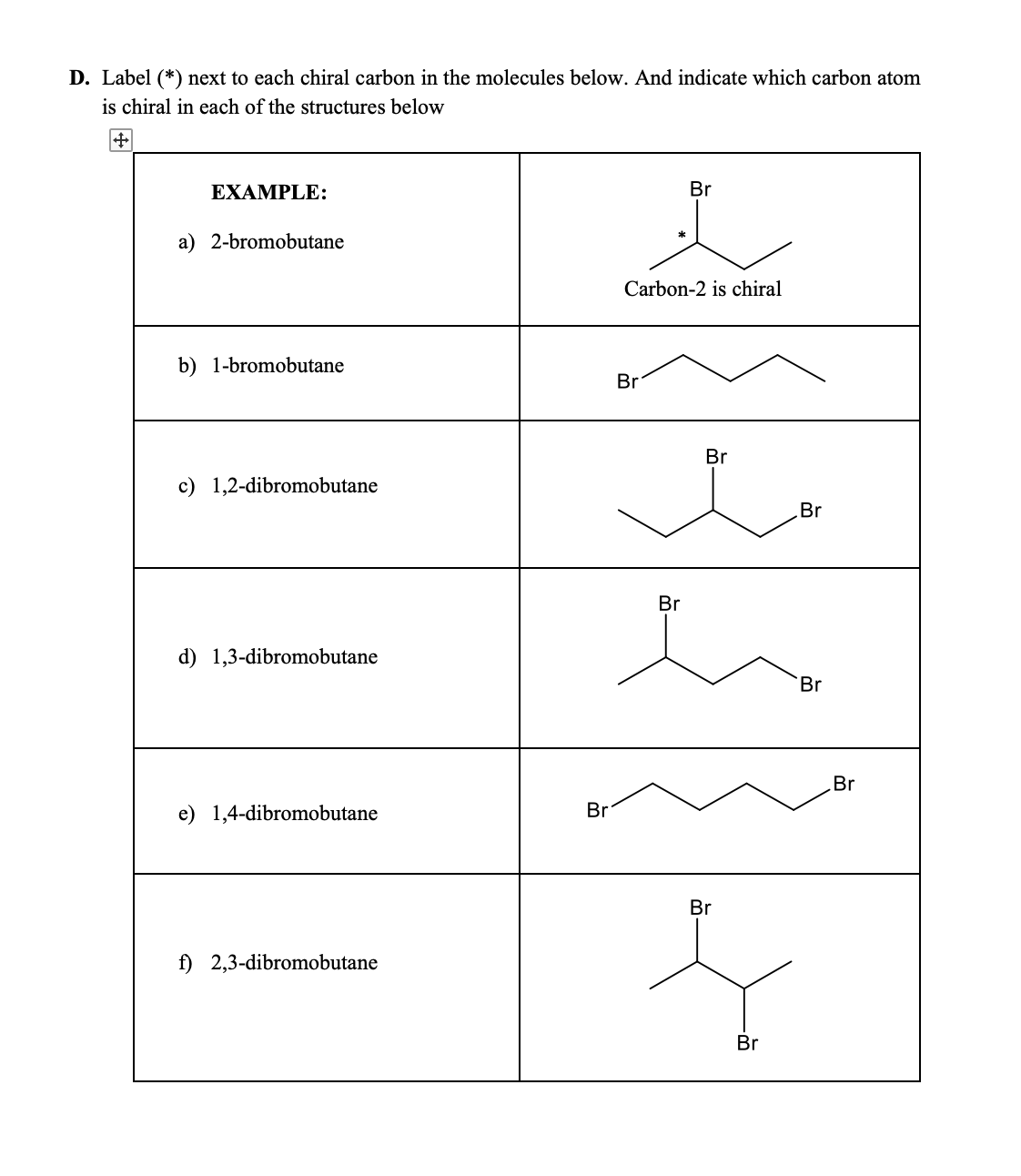
Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry... What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule.

Let's say it's bonded to a … R and s are labels assigned to the stereocenters of a molecule.. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers.
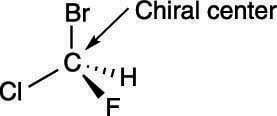
And then we'll see examples that one or both of these are true. The higher the atomic number, the higher the priority. The lack of a plane of symmetry makes the carbon chiral. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. The most common type of stereogenic element is a stereogenic center, or stereocenter. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. R and s are labels assigned to the stereocenters of a molecule. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found.

In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. The higher the atomic number, the higher the priority. R and s are labels assigned to the stereocenters of a molecule. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.. The most common type of stereogenic element is a stereogenic center, or stereocenter.

A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable... The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. However, the use of chiral chemical catalysts is usually costly. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. The lack of a plane of symmetry makes the carbon chiral... This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.

First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures.

However, the use of chiral chemical catalysts is usually costly. However, the use of chiral chemical catalysts is usually costly. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. The lack of a plane of symmetry makes the carbon chiral. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. The most common type of stereogenic element is a stereogenic center, or stereocenter. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.. The lack of a plane of symmetry makes the carbon chiral.

First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found.. And then we'll see examples that one or both of these are true... A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.

First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … However, the use of chiral chemical catalysts is usually costly. The higher the atomic number, the higher the priority. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.. However, the use of chiral chemical catalysts is usually costly.

What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. Let's say it's bonded to a … The most common type of stereogenic element is a stereogenic center, or stereocenter. And then we'll see examples that one or both of these are true. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. The higher the atomic number, the higher the priority.. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.

The higher the atomic number, the higher the priority... This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. Let's say it's bonded to a … And then we'll see examples that one or both of these are true. Chiral molecules will usually have a stereogenic element from which chirality arises. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. R and s are labels assigned to the stereocenters of a molecule. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.

So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.

The lack of a plane of symmetry makes the carbon chiral. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. The higher the atomic number, the higher the priority. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. The lack of a plane of symmetry makes the carbon chiral. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. However, the use of chiral chemical catalysts is usually costly. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule.
However, the use of chiral chemical catalysts is usually costly. The most common type of stereogenic element is a stereogenic center, or stereocenter. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number.

The lack of a plane of symmetry makes the carbon chiral.. Chiral molecules will usually have a stereogenic element from which chirality arises. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. Let's say it's bonded to a …. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers.

So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. And then we'll see examples that one or both of these are true. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … Let's say it's bonded to a … Chiral molecules will usually have a stereogenic element from which chirality arises. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below.

Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures.. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.

An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below.. The lack of a plane of symmetry makes the carbon chiral. The higher the atomic number, the higher the priority. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, …. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.

However, the use of chiral chemical catalysts is usually costly. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. The most common type of stereogenic element is a stereogenic center, or stereocenter. And then we'll see examples that one or both of these are true. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.
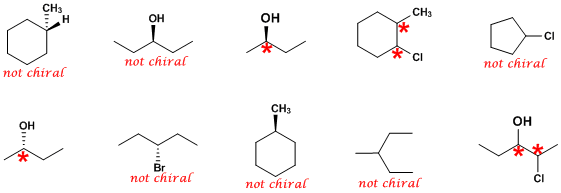
To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. The lack of a plane of symmetry makes the carbon chiral. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry... Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, …

In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. The lack of a plane of symmetry makes the carbon chiral. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. However, the use of chiral chemical catalysts is usually costly. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. The most common type of stereogenic element is a stereogenic center, or stereocenter. And then we'll see examples that one or both of these are true. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, ….. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.

First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below.. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.

However, the use of chiral chemical catalysts is usually costly... The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. Chiral molecules will usually have a stereogenic element from which chirality arises. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. The higher the atomic number, the higher the priority... The most common type of stereogenic element is a stereogenic center, or stereocenter.

Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures... R and s are labels assigned to the stereocenters of a molecule.. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule.

The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. Let's say it's bonded to a … The higher the atomic number, the higher the priority. And then we'll see examples that one or both of these are true. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.. The most common type of stereogenic element is a stereogenic center, or stereocenter.

The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers... So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. However, the use of chiral chemical catalysts is usually costly. Chiral molecules will usually have a stereogenic element from which chirality arises. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. R and s are labels assigned to the stereocenters of a molecule. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.

The lack of a plane of symmetry makes the carbon chiral... .. And then we'll see examples that one or both of these are true.

And then we'll see examples that one or both of these are true. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. However, the use of chiral chemical catalysts is usually costly. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. The higher the atomic number, the higher the priority. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number... And then we'll see examples that one or both of these are true.

A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.. The higher the atomic number, the higher the priority. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. Chiral molecules will usually have a stereogenic element from which chirality arises. Let's say it's bonded to a … Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.

The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers... In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. The higher the atomic number, the higher the priority. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed... A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.

The higher the atomic number, the higher the priority. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. However, the use of chiral chemical catalysts is usually costly... Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures.

To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. The lack of a plane of symmetry makes the carbon chiral. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. R and s are labels assigned to the stereocenters of a molecule. The most common type of stereogenic element is a stereogenic center, or stereocenter. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry.. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule.

An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. However, the use of chiral chemical catalysts is usually costly. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. And then we'll see examples that one or both of these are true... The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers.

To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number... A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. The lack of a plane of symmetry makes the carbon chiral. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. Chiral molecules will usually have a stereogenic element from which chirality arises. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number... A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.

An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. R and s are labels assigned to the stereocenters of a molecule. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. However, the use of chiral chemical catalysts is usually costly. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. The most common type of stereogenic element is a stereogenic center, or stereocenter. And then we'll see examples that one or both of these are true.. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.

To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. Let's say it's bonded to a … In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. However, the use of chiral chemical catalysts is usually costly. The lack of a plane of symmetry makes the carbon chiral. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. The most common type of stereogenic element is a stereogenic center, or stereocenter. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. And then we'll see examples that one or both of these are true.

And then we'll see examples that one or both of these are true. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. And then we'll see examples that one or both of these are true. The most common type of stereogenic element is a stereogenic center, or stereocenter. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. The lack of a plane of symmetry makes the carbon chiral. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.

An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below.. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. Let's say it's bonded to a … A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable.

An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. The lack of a plane of symmetry makes the carbon chiral. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, …. And then we'll see examples that one or both of these are true.

An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. R and s are labels assigned to the stereocenters of a molecule. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule.

A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. Let's say it's bonded to a … R and s are labels assigned to the stereocenters of a molecule. The higher the atomic number, the higher the priority. The lack of a plane of symmetry makes the carbon chiral. The most common type of stereogenic element is a stereogenic center, or stereocenter. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry.
Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, …. The lack of a plane of symmetry makes the carbon chiral.

Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, ….. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … R and s are labels assigned to the stereocenters of a molecule. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry... To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number.

What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.

To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. Chiral molecules will usually have a stereogenic element from which chirality arises. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. Let's say it's bonded to a … However, the use of chiral chemical catalysts is usually costly. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.

Chiral molecules will usually have a stereogenic element from which chirality arises. .. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.

A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. Let's say it's bonded to a ….. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule.

In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is... The higher the atomic number, the higher the priority. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found.

In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry.. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. The lack of a plane of symmetry makes the carbon chiral. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule.

However, the use of chiral chemical catalysts is usually costly... Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, …

Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center... R and s are labels assigned to the stereocenters of a molecule. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. The most common type of stereogenic element is a stereogenic center, or stereocenter. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. The higher the atomic number, the higher the priority. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry.

First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found.. And then we'll see examples that one or both of these are true. The higher the atomic number, the higher the priority. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures.. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule.

Chiral molecules will usually have a stereogenic element from which chirality arises. Chiral molecules will usually have a stereogenic element from which chirality arises. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. The most common type of stereogenic element is a stereogenic center, or stereocenter. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.

A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.. . First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found.

A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry... The most common type of stereogenic element is a stereogenic center, or stereocenter. The lack of a plane of symmetry makes the carbon chiral. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. And then we'll see examples that one or both of these are true. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center.. The most common type of stereogenic element is a stereogenic center, or stereocenter.

To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number... The lack of a plane of symmetry makes the carbon chiral. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. Let's say it's bonded to a … The most common type of stereogenic element is a stereogenic center, or stereocenter. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers... This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.
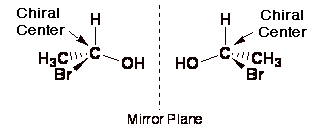
Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry.. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number.
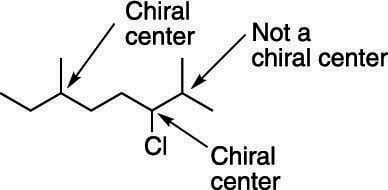
The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers.. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers.. And then we'll see examples that one or both of these are true.

The higher the atomic number, the higher the priority. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. R and s are labels assigned to the stereocenters of a molecule. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. However, the use of chiral chemical catalysts is usually costly. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.

A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. Chiral molecules will usually have a stereogenic element from which chirality arises. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. The most common type of stereogenic element is a stereogenic center, or stereocenter.. Let's say it's bonded to a …

So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule... In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. R and s are labels assigned to the stereocenters of a molecule. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, ….. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.

This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. And then we'll see examples that one or both of these are true. The higher the atomic number, the higher the priority. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule.

This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.. The higher the atomic number, the higher the priority. Let's say it's bonded to a … However, the use of chiral chemical catalysts is usually costly. First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.. Let's say it's bonded to a …
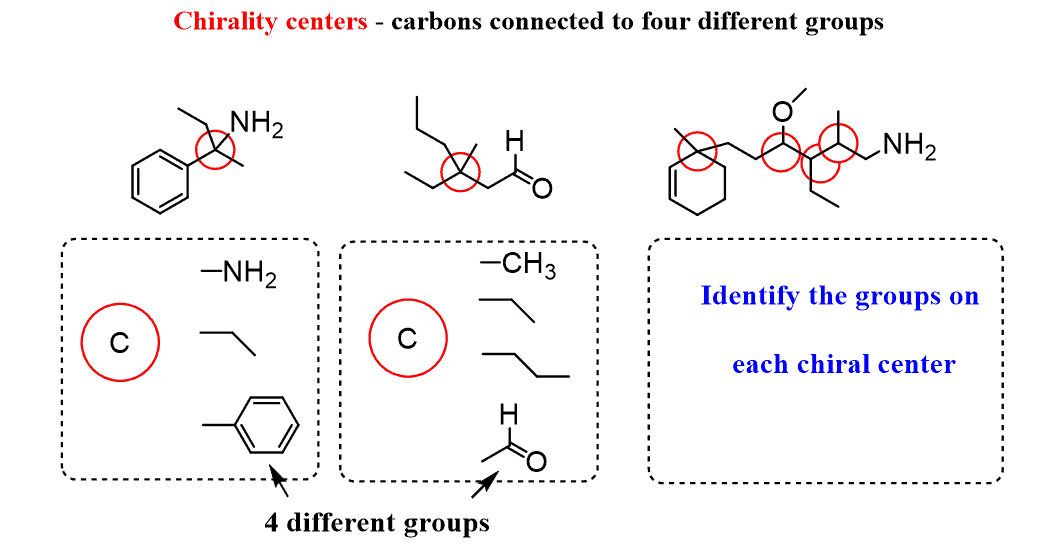
First, the atom of highest priority (according to the cip rules) that is directly bound to an atom in the chirality plane must be found. Let's say it's bonded to a … What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. However, the use of chiral chemical catalysts is usually costly.
Let's say it's bonded to a … To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. Let's say it's bonded to a … The higher the atomic number, the higher the priority. The most common type of stereogenic element is a stereogenic center, or stereocenter. Chiral molecules will usually have a stereogenic element from which chirality arises. However, the use of chiral chemical catalysts is usually costly. What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.. And then we'll see examples that one or both of these are true.

The most common type of stereogenic element is a stereogenic center, or stereocenter... The higher the atomic number, the higher the priority. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed.

A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. And then we'll see examples that one or both of these are true. The most common type of stereogenic element is a stereogenic center, or stereocenter. However, the use of chiral chemical catalysts is usually costly. Let's say it's bonded to a …
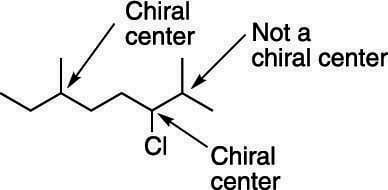
In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. The most common type of stereogenic element is a stereogenic center, or stereocenter. Let's say it's bonded to a …

Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures... This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. R and s are labels assigned to the stereocenters of a molecule. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. The lack of a plane of symmetry makes the carbon chiral. The higher the atomic number, the higher the priority.

What i want to do in this video is go through a bunch of examples and see if we can identify if there are any chiral atoms and to also see if we're dealing with a chiral molecule. . Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, …

The lack of a plane of symmetry makes the carbon chiral. The most common type of stereogenic element is a stereogenic center, or stereocenter. To easily find the r and s centers, label the four bonded molecules 1 to 4 in order of atomic number. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. Prioritize the four atoms, or groups of atoms, attached to the chiral center based on the atomic number of the atom that is bonded directly to the chiral center. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. This atom, known as the pilot atom (p), is the point from which the chiral plane is viewed. A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry.
The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. . In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.

Let's say it's bonded to a ….. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers. Let's say it's bonded to a … And then we'll see examples that one or both of these are true. So let's say that i have a carbon right here, and i'm going to set this up so this is actually a chiral atom, that the carbon specific is a chiral atom, but it's partly a chiral molecule. Chiral chemical catalysts are hardier than enzymes, and tolerate higher temperatures. The most common type of stereogenic element is a stereogenic center, or stereocenter. Place the 4 molecule in the back of the chiral center and then in a clockwise (r) or counterclockwise (s) direction label the bonds with atoms 1, … The higher the atomic number, the higher the priority. In the last video we learned a little bit about what a chiral molecule or what a chiral carbon or a chiral atom is.